Keeping your business open and safe during COVID-19
Partner with us for COVID-19Experience
For the detection of COVID-19, the Everlywell solution can play an essential role in keeping participants safe, customers happy and business moving forward.

Comprehensive
One end-to-end digital solution to manage the complete COVID-19 testing process

Trusted
We partner with CLIA-certified and CAP-accredited labs. Our platform is HIPAA-compliant
Easy & Painless
Simple instructions and effortless collection method
Physician Reviewed
A telehealth consult is included for those with positive COVID-19 results
Best in Class
The Everlywell COVID-19 Test Home Collection Kit is unmatched

Turnaround time
Quick shipment. Test results typically provided within 24-48 hours of the lab receiving the sample.

Ability to scale
Our FDA EUA enables us to partner with multiple labs, answering demand.

Reliable
The Everlywell RT-PCR nasal swab is one of the most reliable methods for COVID-19 detection.
In the news

“From a simplicity standpoint, we have to get through…100 percent of the student body that is in residence. There is no way that we can do that, having even all of the health professionals in this college working at the same time...Everlywell was able to meet the College’s high demand for tests, whereas other vendors were not." -The Kenyon Collegian

"I had initially expected to prefer a saliva-based collection method versus a nasal swab but ultimately ended up much preferring the nasal swab. The nasal swab took me less than five minutes, and contrary to early reports did not hurt at all." - Health.com
For organizations ordering 500 or more collection kits
Our digital platform handles the details so you don’t have to

Test distribution & registration is simple with multiple shipping options to meet your needs.

Regular updates provide visibility into the testing process. Participants can receive alerts via text message.

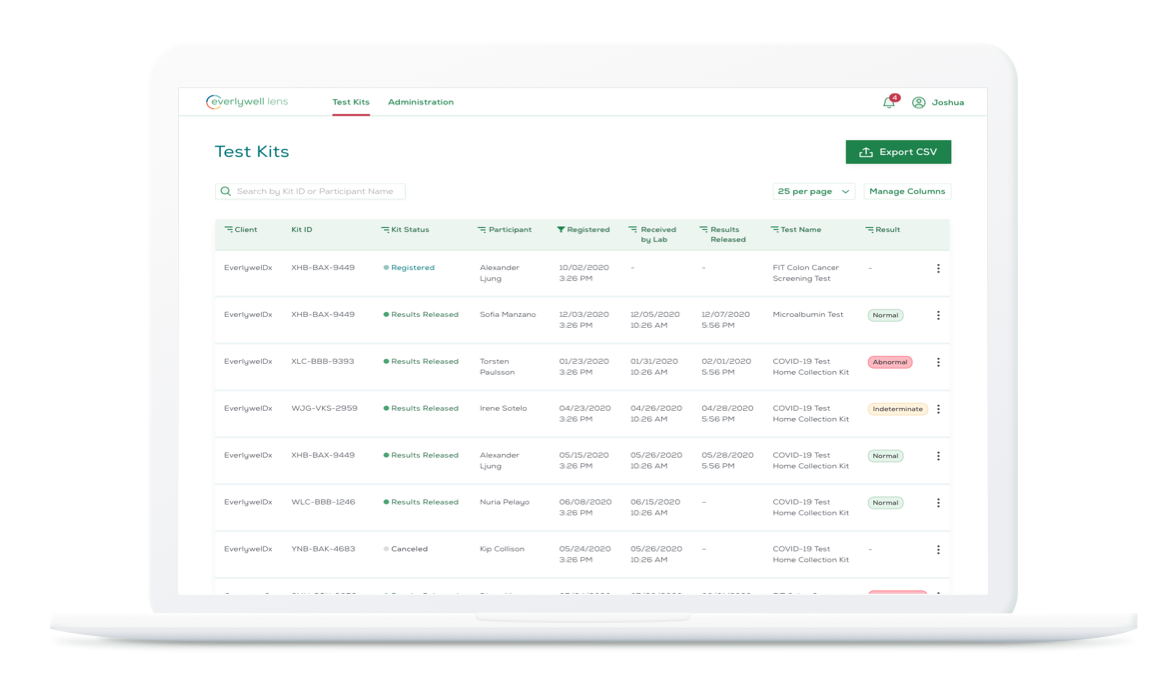
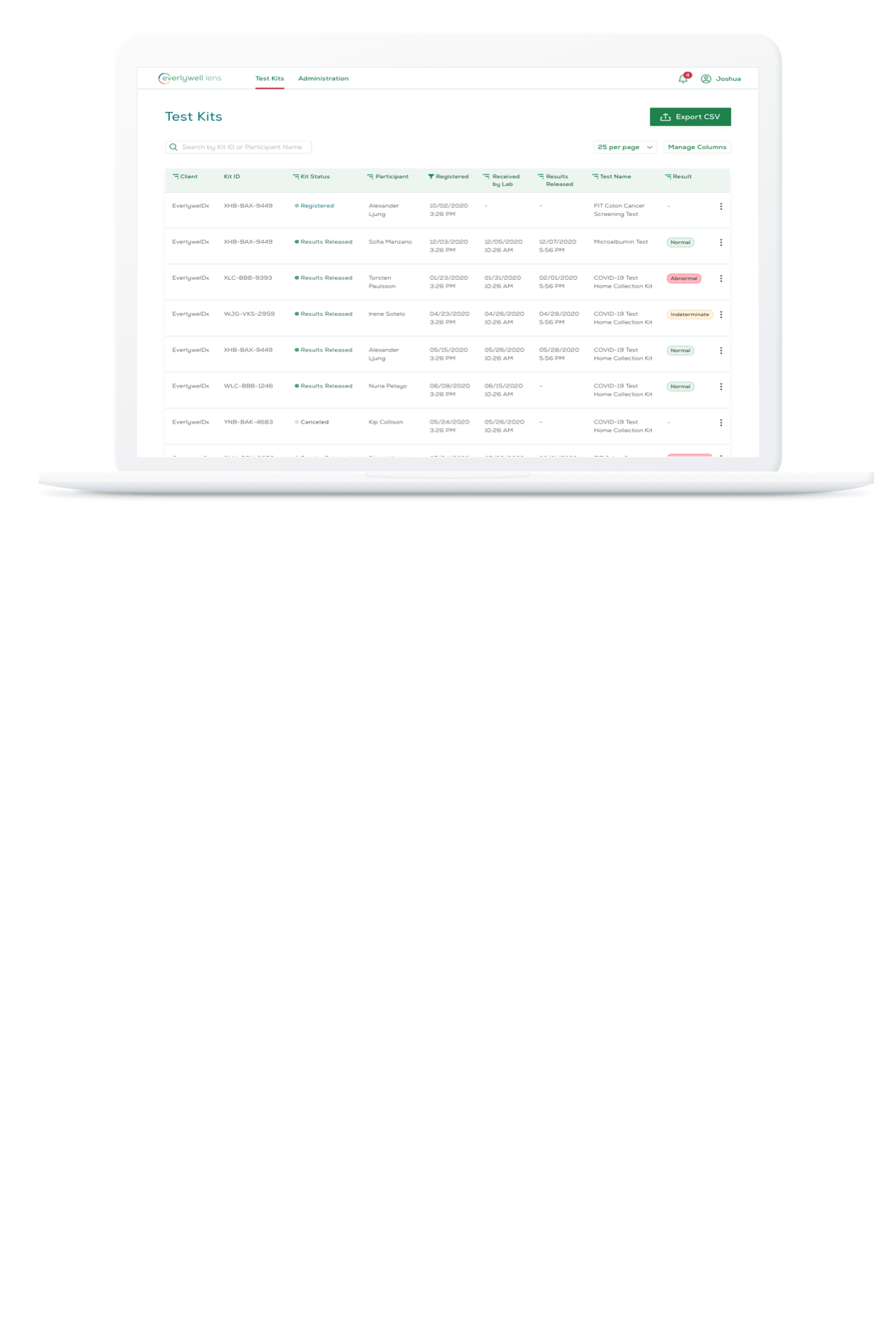
The digital dashboard, Everlywell Lens, enables organizations to review individual and aggregated results, alerting in real-time of abnormal results.
Are you ready to partner with us?
Notes:
- This home collection kit has not been FDA cleared or approved.
- This home collection kit has been authorized by FDA under an EUA.
- This home collection kit has been authorized only for the home collection and maintenance of nasal swab specimens as an aid in detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens.
- This home collection kit in combination with the authorized test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.